FAQs
Answers to the most frequently asked questions on biosimilar medicines
What is a biological medicine?
Biological medicines are highly sophisticated medicines made from living cells or organisms (like bacteria and yeast).
What makes biological medicines different to other medicines?
To understand how biological medicines differ from other medicines, it helps to compare them to more common medicines that you may be familiar with, those made by chemical processes.
| Chemical medicines | Biological medicines | |
| Example | Aspirin | Infliximab |
| What do they look like under a microscope? | Small and simple, made up of 21 atoms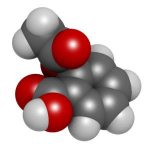 | Very large and highly complex, made up of more than 20,000 atoms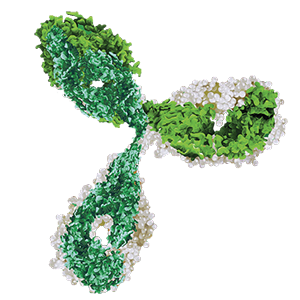 |
| How are they made? | Simple chemical process | Complex biological process using the latest technology to modify the genes to produce the active ingredients for the medicine |
| How do you take them? | Commonly taken as pills | Cannot be made into a pill and are given as an injection or infusion instead |
| Can we make copies? | Yes, the active ingredient will be 100% identical across all batches | No, we cannot make identical copies of biological medicines. It’s normal to find minor differences between batches of the same biological medicine |
| What are new brands of the same medicine called? | Generic medicine | Biosimilar medicines |
Are biological medicines safe?
Yes. All biological medicines are thoroughly tested and must meet the strict standards of the Therapeutic Goods Administration (TGA) for quality, safety and effectiveness.
What is a biosimilar medicine?
A biosimilar medicine is a highly similar version of an original brand of a biological medicine, marketed by a different manufacturer once the patent on the original brand expires.
How much experience do we have with biosimilar medicines?
Biosimilar medicines have been used since 2006, with Europe being the first region to approve them. Their use has steadily increased over time and they are currently available in more than 60 countries around the world. Biosimilar medicines have been available in Australia since 2010, where they’ve helped improve the lives of thousands of Australians with serious diseases such as cancer, rheumatoid arthritis, inflammatory bowel disease, severe psoriasis, multiple sclerosis and kidney disease.
Is a biosimilar medicine the same as a generic?
No, generic medicines are identical to the original brand because they are produced by chemical processes. A biosimilar medicine is a highly similar version of an original brand of a biological medicine. This is because – just like in nature, where no two things are ever identical copies – there are natural differences between all biological medicines.
Is a biosimilar medicine as safe and effective as the original brand?
Yes. To be available in Australia, all biosimilar medicines are thoroughly tested and must meet the high standards of the Therapeutic Goods Administration (TGA). This ensures that they meet the same strict standards for quality, safety and effectiveness as the original brand. Any minor differences between the original and the biosimilar medicine have been shown to have no difference for the person who requires the medicine (i.e. no difference in safety or effectiveness).
Why did my pharmacist offer me a different brand of biological medicine?
If your doctor doesn’t specify the brand of biological medicine on your prescription, then your pharmacist is able to offer you an alternative brand. The pharmacist must explain this to you and ensure that you’re happy with the change. At the end of the day, it’s up to you and your doctor to decide which brand of biological medicine is right for you.
Are there any differences I need to be aware of between brands of biological medicines?
There may be differences in how the device works between brands of biological medicines that you administer yourself i.e. the pen or auto-injector. It’s essential that your healthcare team show you how your new device works. Be sure to ask if you have any questions or concerns.
I get my treatment in a public hospital; do I have a choice about which brand of biological medicine I receive?
This depends on your hospital. Some hospitals have decided to hold only one brand of a biological medicine – this may be the original brand or the biosimilar brand for that medicine – while other hospitals may offer multiple brands. Be sure to discuss any questions with your healthcare team.
What is the benefit of biosimilar medicines?
In Australia, we benefit from a world class healthcare system where the Government ensures medicines and health services remain affordable to those who need them. For example, under the Pharmaceutical Benefit Scheme (PBS), a medicine that costs $1,000 will only cost $40.30 (or $6.50 for concession) at the pharmacy. With the increasing availability of biological medicines for more diseases, the Government is spending a significant portion of the medicines budget on keeping biological medicines affordable for those who need them.
The availability of biosimilar medicines contributes to the sustainability of the Australian healthcare system by increasing competition between brands of the same biological medicine, leading to reduced overall costs for the healthcare system. By reducing the cost of healthcare services, the Government can invest in new medicines and offer better treatments to more people who need them, ensuring Australians receive world class healthcare today and in the future.
Will a biosimilar medicine save me money?
The savings from biosimilar medicines are felt by Australia as a whole through cost savings for the public healthcare system. You pay the standard PBS co-payment whether you receive the original or the biosimilar brand of biological medicine (i.e. $40.30 or $6.50 for concession).
What is the benefit of using a biosimilar medicine over the original brand of a biological medicine?
You will experience the same safety and effectiveness from your biological medicine whether you receive the original brand or the biosimilar brand. The benefits of biosimilar medicines are for the wider Australian population and future generations through cost savings for the public healthcare system.
Where can I find more information?
Your healthcare team is always your best source of information as they understand your individual needs. Don’t hesitate to ask them if you have any questions or concerns.
For information on a specific biological medicine, you can access the Consumer Information Leaflets on the TGA website here.
Our Useful Links page lists additional websites that we hope you find useful.
